Company
Yuqiang Intelligent - Providing ultimate service to global customers
Creating ultimate products with ultimate technology, ultimate craftsmanship, ultimate cost, and ultimate quality
A high-tech enterprise founded by technical and management elites with rich experience in the pharmaceutical machinery industry, supported by a strong A-share listed company
The company is mainly engaged in the research and development, manufacturing, sales and service of pharmaceutical equipment.

Products
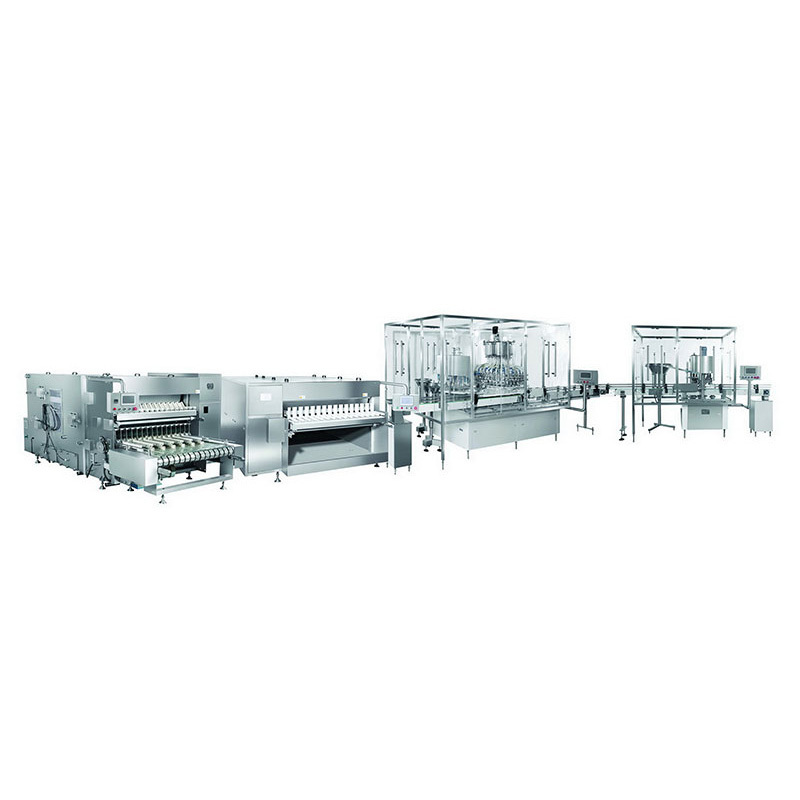
Product Catalog
Oral liquid production line
Ampoule production line
Plastic bottle production line
Soft bag production line
Glass bottle production line
News
Always pay attention to news trends
 2025-04-25
2025-04-25
From Sichuan! This innovative medical device has been approved for market launch→
 2025-04-25
2025-04-25
Businesses can respond to classification in the following ways
 2025-04-25
2025-04-25
How to cope with medical device classification rules